Many Active Pharmaceutical Ingredients (APIs) intended for intravenous use are poorly soluble or insoluble in water, creating significant development challenges. Poor solubility can cause promising drugs to fail in development and limit the use of approved drugs. Estimates suggest that 70–90% of drugs in development and about 40% of approved drugs are classified as poorly soluble.
Common strategies to improve solubility — such as using solvents like polymers, polyoxyl oils, and ethanol — can cause acute or delayed side effects, sometimes serious. Such side effects have been seen as an unpleasant trade off in cancer treatment and may require corticosteroid premedication and slow infusion times, limiting patient flow in busy chemotherapy suites.
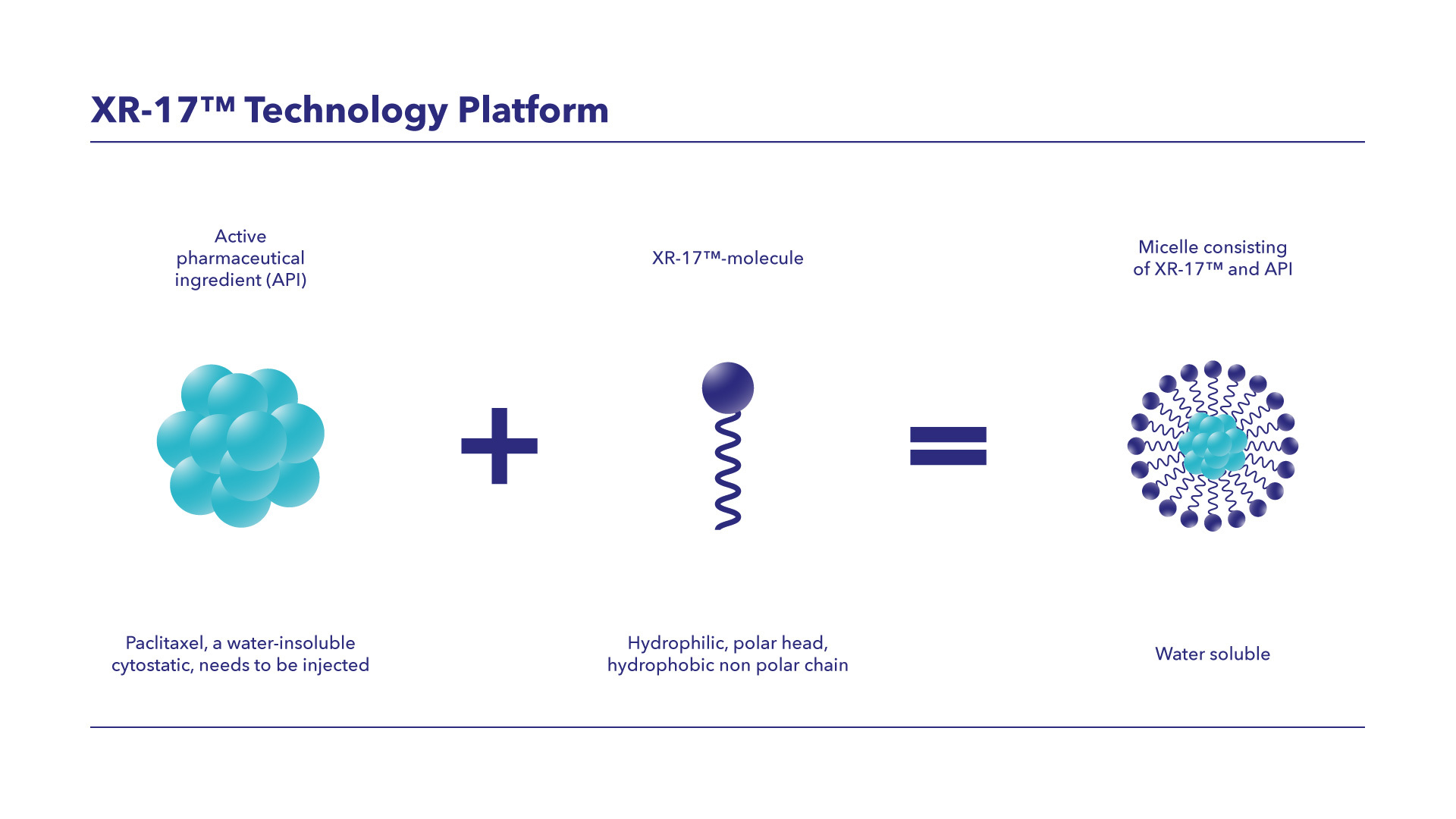
To meet this medical need and help improve the efficiency of the drug development process, Vivesto developed and patented XR-17, a first-generation drug delivery platform that enhances the solubility of intravenous substances and enables innovative API formulations.
Combining the XR technology platform with effective APIs allows Vivesto to create innovative, patent-protected drugs that benefit patients. This technology was successfully used to develop Apealea.
XR-17 is based on a mix of two vitamin A derivatives. It forms micelles with APIs, which are 20–60 nanometers in diameter. A human hair is ~80,000–100,000 nanometers.
Poorly water-soluble molecules are enclosed in the micelle core and released into the bloodstream upon administration. Because XR-17 is well-tolerated, it enables treatment with otherwise insoluble substances without the risks typically associated with solvent use (e.g., hypersensitivity reactions).
Vivesto is also developing a next-generation platform, XR-18, and is actively seeking partners for further development.